Program Overview
Important Dates
Innovation Themes
Eligibility Requirements
Selection Criteria
Role of the Host Institution
Funding
Process and Timeline
Webinars
Resources
Program Overview
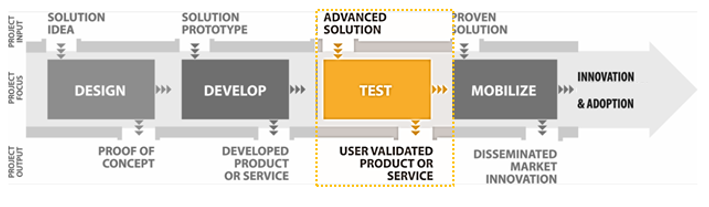
The Centre for Aging + Brain Health Innovation’s (CABHI) mission is to accelerate the development, validation, commercialization, dissemination and adoption of innovative products, services and best practices to support brain health and aging. Our vision is a world in which people can age in the setting of their choice, maintaining their well-being and independence for as long as possible.
The Researcher-Clinician Partnership Program (RCP2) will support teams of clinicians and researchers collaborating to refine, test, validate and disseminate innovative solutions in real-world settings. Solutions must be at an advanced stage of development, with promising scalability. Organizations of successful applicants will receive up to $500,000 (CAD) to support their projects. Projects cannot exceed 15 months in duration (including submission of final project report to CABHI) and must begin by August 2018.
Interested applicants must submit an online application no later than 5pm on May 18th, 2018.
Important Dates
| RCP2 Announcement | April 2, 2018 |
| Applicants submit online application including letter of support | May 18, 2018 (by 5:00 PM EST) |
| Applicants notified | June 18, 2018 |
| Sign agreements | July 31, 2018 |
| Start project | August 2018 |
Eligibility Requirements
Innovation Themes
CABHI supports innovations that address challenges associated with the aging brain, including dementia and other neurodegenerative diseases. In 2018, CABHI’s focus will be on testing innovative solutions that are aimed at addressing the specific themes listed below. All project applications must focus on one or more of these themes. View CABHI’s priority themes.
- Aging in place: solutions that enable older adults with dementia to maximize their choice, independence and quality of life regarding where they live
- Caregiver support: solutions that support caregivers (formal and informal) in providing care to older adults with dementia
- Care coordination and navigation: solutions that help older adults, caregivers and healthcare providers coordinate care and transitions for older adults with dementia
- Cognitive health: solutions focused on health promotion, prevention, early diagnostics, and slowing the progression of cognitive impairment in aging adults
Applicants
Applicants must apply as a team composed of at least one researcher and one clinician working jointly on study design, trial implementation, data collection, analysis and reporting. Team members do not have to be employed by the same organization.
Either the lead clinician or lead researcher must be identified as the project’s Principal Investigator (PI). The PI must be employed by an institution that is a Canada Revenue Agency (CRA) “qualified donee” located in Canada. This institution will act as the project’s Host Institution (see section 4 – Role of Host Institution, below).
Teams may also include collaborators from other organizations. Only the Host Institution must be a CRA qualified donee.
Projects
The project must focus on evaluating the application and impact of the solution in real-world seniors’ care settings, using rigorous scientific methodology but going beyond lab tests and other tightly-controlled test environments.
The initiative cannot involve basic research, primarily academic goals, capital investment in a portfolio of intellectual property, pharmaceutical clinical trials or incubators for startups.
Solutions
The Intellectual Property in the solution being tested must be owned by (i) the Host Institution, (ii) the researcher or clinician who is employed by the Host Institution and identified as the Principal Investigator on the project, or (iii) a combination of the above. In all cases, the Host Institution must have the right to use the Intellectual Property during the course of the project.1
Solutions must, at minimum, be advanced ready for demonstration in an appropriate operational environment for the duration of the proposed trial.
The solution must be “innovative” in that it deviates from standard practice or common procedure and demonstrates purposeful differentiation from existing widely adopted solutions.
1 Note: If the Principal Investigator owns all or a portion of the Intellectual Property, he or she may be required by CABHI to match CABHI’s contributions through cash and/or in-kind support.
Selection Criteria
All applications will be evaluated using the following criteria:
Applicant information (20% of total score)
- Team has a demonstrated track record of relevant successes and appropriate qualifications to undertake the trial
- Host Institution has a track record of implementing and mobilizing innovations, capacity and expertise to undertake research, provides evidence of strong local support
Innovative product, service or practice (“Solution”) (20% of total score)
- Clear description of the solution’s key features, qualities and how it is used
- Clear and convincing description of the benefits of the solution and how it improves the current status quo (such as outcomes, efficiencies and cost savings); presents compelling and clear evidence (in the form of scientific evidence and/or user testing) to support claims
- Solution is at a current state of development (including licensing and certifications) to be used in an operational setting
Dissemination, adoption and future financial sustainability (20% of total score)
- Clear plan to disseminate and adopt solution by clinicians and/or other end users, including an analysis of target audiences, anticipated barriers, and how to address them
- Solution can be scaled to impact a large number of users regionally, nationally or globally; Has the applicant adequately identified and addressed requirements to scale?
- Where applicable: clear strategy for financial sustainability, including target market segments and competitive landscape analysis; plan includes financial assumptions and supporting data
Strategic Alignment (20% of total score)
- Convincing description of how the solution specifically addresses an important, and concrete problem faced by the health system and/or older adults, and how it aligns with at least one of CABHI’s priority themes; supported by data from previous research completed in simulated environment. View CABHI’s priority themes.
Project Overview & Research Approach (20% of total score)
- Clear and concise overview of the project provided, including the main goals and activities to be funded as part of this project and the key questions the research will answer
- Approach to data collection and analysis is well designed given the research questions being addressed and project metrics being captured; reasonable outcome targets are identified
- Project is well planned and sufficiently resourced
- Project budget leverages contributions from other sources through cash or in-kind contributions
Online applications will be reviewed and scored by a project evaluation panel that is comprised of an independent group of external reviewers with expertise in discovery, clinical and/or applied research, business and technical fields.
Recommendations from the project evaluation panel will be considered by CABHI leadership for project selection to ensure balance and strategic alignment.
CABHI reserves the right to select proposals that represent a distribution of projects across the 2018 innovation themes and geographic spread across Canada.
CABHI reserves the right to decline any application and to modify or annul this Call for Innovations at any time, without incurring any liability.
Role of the Host Institution
The Host Institution has a number of key responsibilities:
- It will be responsible for receiving, and administering the funding.
- It will be accountable for the completion of project milestones and reporting deliverables.
- It will enter into a contract with CABHI detailing the roles, responsibilities, accountabilities and reporting requirements for the project. If applicable, the Host Institution will also be responsible for contracting with other institutions employing team members on the project.
- It will be responsible for distributing funds received from CABHI to support the activities of project team members at their partner organizations. CABHI reserves the right to require other members of the project team to execute agreements in connection with the project.
- It will provide facilities, management and support (direct and indirect) for the project team so that they can effectively and efficiently engage in the proposed project.
- It will ensure compliance with, and oversight of, applicable regulatory and licensing requirements, and best practices in research related to recruitment, consenting, privacy, data security, ethics and training.
- Where applicable, it will support and share project costs related specifically to the trial.
- It will provide the project team with access to a population of test subjects by either drawing on its own client base or drawing on the client base(s) of the organization(s) collaborating on the project.
Funding
Funding from CABHI
CABHI will support project costs that are directly associated with the trial of an innovative solution (product, service, or practice), to a maximum of CAD $500,000 with a 15-month timeline for completion (including submission of final project report). Refer to the CABHI Program Eligible Expenses Guideline for information on eligible project costs.
CABHI funding will be provided to the Host Institution employing the PI. The Host Institution is responsible for distributing funds to other project participants.
Funding from the Host Institution
No specific amount of matching funds from the Host Institution is required to apply for the RCP2. However, preference will be given to applications that demonstrate how CABHI’s funding will leverage support from other funding sources.
For example, applicants might show evidence of government, collaborator or institutional commitment through a blend of cash and/or in-kind contributions such as office or lab space, equipment, connectivity, staff salaries, etc.
Flow of Funds from CABHI
CABHI will release to the Host Institution an initial payment of 30% of the approved project budget at the start of the project.
Two subsequent interim payments of 30% of the approved project budget will be released to the Host Institution when project milestones and deliverables have been met and progress and financial reports have been received from the Host Institution.
A holdback of 10% of the project budget will be released to the Host Institution upon project completion when project milestones and deliverables have been met and upon receipt of the final progress report, financial report, and attestation regarding the use of funds from the Host Institution.
The Host Institution must provide interim and final financial reports on use of project funds.
Ownership Rights
CABHI will not retain any ownership rights to the intellectual property associated with the solution (product, service, or practice) but will retain the right to disseminate the findings of the testing completed.
If and when the Innovation is commercialized (whether through the licensing, sale or other exploitation of the innovation), and if and when the Host Institution receives a royalty or any other form of payment in connection with such commercialization, then CABHI shall have the right to receive a reasonable royalty or payment from the Host Institution, in an amount to be negotiated by the Parties.
Process and Timeline
| RCP2 Announced | April 2, 2018 |
| Step 1: Applicants submit online application (including letter of support) | May 18, 2018 (by 5:00 PM EST) |
| Online application must be submitted using the online application by 5:00 PM EST on May 18, 2018. All applications must be in English and cannot contain confidential or proprietary material. All applications must include a letter of support signed by VP level executive from the Host Institution. | |
| Step 2: CABHI notifies selected applicants | June 18, 2018 |
| After being reviewed for eligibility, submitted applications will be scored by external subject matter experts (Applicants may be contacted for additional information during the evaluation phase). CABHI leadership reviews final rankings and selects successful projects. | |
| Step 5: CABHI and Signatories sign agreements | No later than July 31, 2018 |
| The agreement will set out any contributions/obligations of the Host Institution, and any other Signatories along with the rights of CABHI, including rights to disseminate the findings. If the agreement is not signed in a reasonable amount of time (determined exclusively by CABHI), CABHI may terminate discussions, terminate the program or select an alternate applicant. | |
| Start project | August 2018 |
Webinars
Join one of our webinars to learn more!
Register for one of our webinars to learn more about how to submit a successful proposal for RCP2. Topics will include: keys to a successful proposal, how to submit a proposal through our online portals, funding allocation and responsibility for reporting, and much more.
Webinar Dates & Times:
Register for one of our webinars to learn more about how to submit a successful proposal for RCP2. Topics will include: keys to a successful proposal, how to submit a proposal through our online portals, funding allocation and responsibility for reporting, and much more.
Fri, Apr 13, 2018 1:00 PM – 2:00 PM EDT (Link to register)
Fri, Apr 27, 2018 1:00 PM – 2:00 PM EDT (Link to register)
Resources
In-Kind Contributions & Matching Funds Guideline
View the 2018 Researcher-Clinician Partnership Program – Round 3 Call for Innovations in French.
Funding for this program is provided by the Government of Ontario through the Ministry of Research, Innovation, and Science, as well as the Government of Canada through the Public Health Agency of Canada and the Baycrest Foundation.